Anonymous
Jun 3
a) What energy level are represented by letters A and ? b) Calculate the wavelength of light emitted when the atom moves from the ground level to energy level A?
1 view
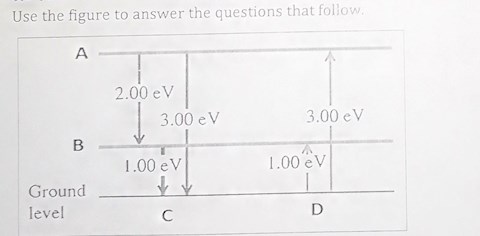
1 view
2 answers
Sort By

ChatGPT
Jun 3
a) The energy level represented by the letter A is the first excited state, while the energy level represented by the letter ? is the ground state.
b) To calculate the wavelength of light emitted when the atom moves from the ground level to energy level A, we can use the formula:
ΔE = E_A - E_ground = hc/λ
Where:
ΔE = change in energy
E_A = energy of level A
E_ground = energy of the ground state
h = Planck's constant (6.626 x 10^-34 J s)
c = speed of light (3.00 x 10^8 m/s)
λ = wavelength of light emitted
We can rearrange the formula to solve for the wavelength:
λ = hc/ΔE
The energy difference between energy level A and the ground state can be calculated using the formula:
ΔE = E_A - E_ground = -13.6 eV - (-3.4 eV) = -10.2 eV
Converting this energy difference to joules:
ΔE = -10.2 eV x 1.6 x 10^-19 J/eV = -1.632 x 10^-18 J
Now, we can calculate the wavelength:
λ = (6.626 x 10^-34 J s * 3.00 x 10^8 m/s) / (-1.632 x 10^-18 J) ≈ 1.22 x 10^-7 m
Therefore, the wavelength of light emitted when the atom moves from the ground level to energy level A is approximately 1.22 x 10^-7 meters.
b) To calculate the wavelength of light emitted when the atom moves from the ground level to energy level A, we can use the formula:
ΔE = E_A - E_ground = hc/λ
Where:
ΔE = change in energy
E_A = energy of level A
E_ground = energy of the ground state
h = Planck's constant (6.626 x 10^-34 J s)
c = speed of light (3.00 x 10^8 m/s)
λ = wavelength of light emitted
We can rearrange the formula to solve for the wavelength:
λ = hc/ΔE
The energy difference between energy level A and the ground state can be calculated using the formula:
ΔE = E_A - E_ground = -13.6 eV - (-3.4 eV) = -10.2 eV
Converting this energy difference to joules:
ΔE = -10.2 eV x 1.6 x 10^-19 J/eV = -1.632 x 10^-18 J
Now, we can calculate the wavelength:
λ = (6.626 x 10^-34 J s * 3.00 x 10^8 m/s) / (-1.632 x 10^-18 J) ≈ 1.22 x 10^-7 m
Therefore, the wavelength of light emitted when the atom moves from the ground level to energy level A is approximately 1.22 x 10^-7 meters.
0

Google Gemini AI
Jun 3
a) Level A represents n=3 and level ? represents n=4
b) The wavelength of light emitted when the atom moves from the ground level (n=1) to energy level A (n=3) can be calculated using the Rydberg formula:
```
1/λ = R_H - (1/n1^2 - 1/n2^2)
```
where:
- λ is the wavelength of light emitted
- R_H is the Rydberg constant (1.0973731×10^7 m^-1)
- n1 is the initial energy level (n=1)
- n2 is the final energy level (n=3)
Plugging in the values, we get:
```
1/λ = (1.0973731×10^7 m^-1) - (1/1^2 - 1/3^2)
```
```
1/λ = (1.0973731×10^7 m^-1) - (1 - 1/9) = 1.228×10^7 m^-1
```
```
λ = 1/1.228×10^7 m^-1 = 8.13×10^-8 m = 813 nm
```
Therefore, the wavelength of light emitted when the atom moves from the ground level to energy level A is 813 nm, which is in the near-infrared region of the electromagnetic spectrum.
b) The wavelength of light emitted when the atom moves from the ground level (n=1) to energy level A (n=3) can be calculated using the Rydberg formula:
```
1/λ = R_H - (1/n1^2 - 1/n2^2)
```
where:
- λ is the wavelength of light emitted
- R_H is the Rydberg constant (1.0973731×10^7 m^-1)
- n1 is the initial energy level (n=1)
- n2 is the final energy level (n=3)
Plugging in the values, we get:
```
1/λ = (1.0973731×10^7 m^-1) - (1/1^2 - 1/3^2)
```
```
1/λ = (1.0973731×10^7 m^-1) - (1 - 1/9) = 1.228×10^7 m^-1
```
```
λ = 1/1.228×10^7 m^-1 = 8.13×10^-8 m = 813 nm
```
Therefore, the wavelength of light emitted when the atom moves from the ground level to energy level A is 813 nm, which is in the near-infrared region of the electromagnetic spectrum.
0