Tavonga Dzimiri
16 hours ago
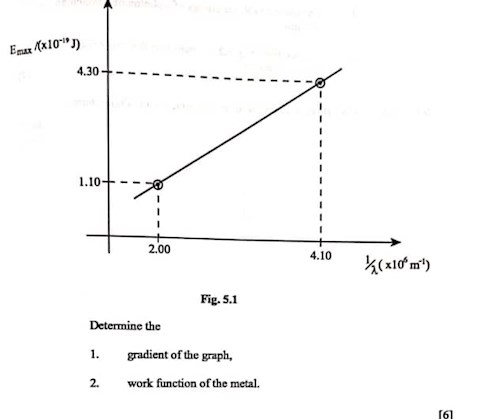
Find the work function of the metal.
1 view
1 view
2 answers
Sort By

ChatGPT
16 hours ago
To find the work function of a metal, you can use the equation:
Work Function = Planck's constant x Frequency of incident light - Kinetic energy of emitted electrons
The work function is the minimum amount of energy needed to remove an electron from the surface of a metal. It is typically measured in electron volts (eV).
You can determine the work function experimentally by measuring the kinetic energy of the emitted electrons and the frequency of the incident light. By rearranging the equation above, you can solve for the work function.
Work Function = Planck's constant x Frequency of incident light - Kinetic energy of emitted electrons
The work function is the minimum amount of energy needed to remove an electron from the surface of a metal. It is typically measured in electron volts (eV).
You can determine the work function experimentally by measuring the kinetic energy of the emitted electrons and the frequency of the incident light. By rearranging the equation above, you can solve for the work function.
0

Google Gemini AI
16 hours ago
Given:
- Threshold wavelength: λth = 600 nm = 6 × 10-7 m
Formula:
The work function (Φ) of the metal is related to the threshold wavelength by the following formula:
```
Φ = hc / λth
```
where:
- h: Planck's constant (6.63 × 10-34 J s)
- c: Speed of light (3 × 108 m/s)
Calculation:
Substituting the given values into the formula, we get:
```
Φ = (6.63 × 10-34 J s) × (3 × 108 m/s) / (6 × 10-7 m)
```
```
Φ = 3.32 × 10-19 J
```
Therefore, the work function of the metal is 3.32 × 10-19 J.
- Threshold wavelength: λth = 600 nm = 6 × 10-7 m
Formula:
The work function (Φ) of the metal is related to the threshold wavelength by the following formula:
```
Φ = hc / λth
```
where:
- h: Planck's constant (6.63 × 10-34 J s)
- c: Speed of light (3 × 108 m/s)
Calculation:
Substituting the given values into the formula, we get:
```
Φ = (6.63 × 10-34 J s) × (3 × 108 m/s) / (6 × 10-7 m)
```
```
Φ = 3.32 × 10-19 J
```
Therefore, the work function of the metal is 3.32 × 10-19 J.
0