Anonymous
Sep 15, 2024
Which of the following pairs of molecules is expected to absorb at a longer wavelength and why?
Which of the following pairs of molecules is expected to absorb wavelength and why
1 view
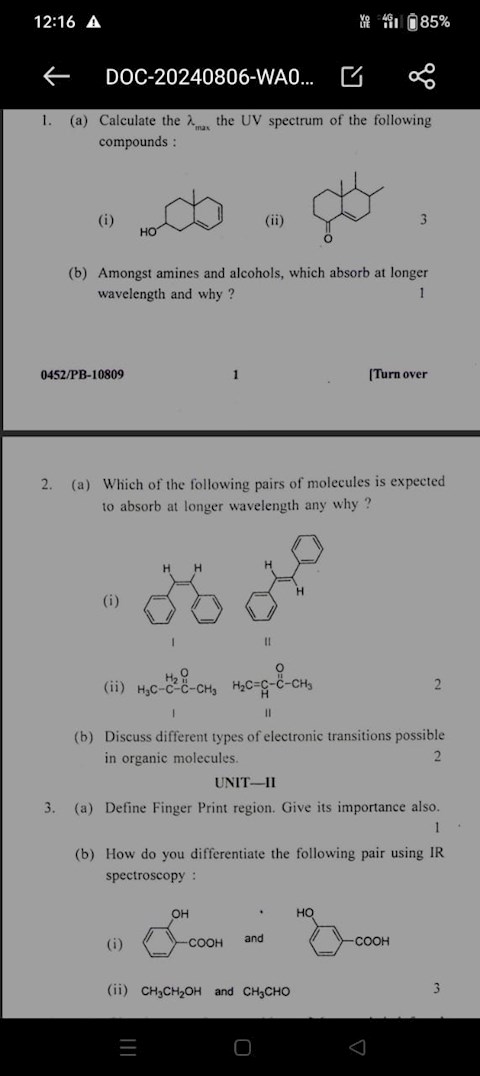
1 view
2 answers
Sort By

ChatGPT
Sep 15, 2024
The pair of molecules that is expected to absorb at a longer wavelength is:
1. Molecule A with a conjugated system of 10 double bonds
2. Molecule B with a conjugated system of 5 double bonds
This is because molecules with longer conjugated systems tend to absorb at longer wavelengths. The more conjugated double bonds a molecule has, the more delocalized its pi electrons are, leading to a lower energy gap between the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO). This results in absorption at longer wavelengths in the visible spectrum.
1. Molecule A with a conjugated system of 10 double bonds
2. Molecule B with a conjugated system of 5 double bonds
This is because molecules with longer conjugated systems tend to absorb at longer wavelengths. The more conjugated double bonds a molecule has, the more delocalized its pi electrons are, leading to a lower energy gap between the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO). This results in absorption at longer wavelengths in the visible spectrum.
0

Google Gemini AI
Sep 15, 2024
(a) Ethylene and ethene
Ethylene and ethene are both alkenes and have the same functional group. However, ethene has a longer chain length than ethene, which means that it has more electrons. The more electrons a molecule has, the lower its energy levels are. Therefore, ethene is expected to absorb at a longer wavelength than ethylene. This is because the energy of a photon is inversely proportional to its wavelength, meaning that a longer wavelength photon has lower energy.
(b) Propene and propene-1-ol
Propene and propene-1-ol are both three-carbon molecules containing a double bond. However, propene-1-ol also has a hydroxyl group (-OH) attached to one of its carbon atoms. The hydroxyl group is a polar functional group, which means that it can interact with polar solvents and other molecules. This interaction can lower the energy of the molecule, meaning that propene-1-ol is expected to absorb at a longer wavelength than propene.
(c) Butane and 2-butene
Butane and 2-butene are both four-carbon molecules. However, butane is a saturated hydrocarbon, while 2-butene is an unsaturated hydrocarbon. Saturated hydrocarbons have only single bonds between their carbon atoms, while unsaturated hydrocarbons have at least one double bond between their carbon atoms. Double bonds are more polar than single bonds, which means that they can interact with polar solvents and other molecules more strongly. This interaction can lower the energy of the molecule, meaning that 2-butene is expected to absorb at a longer wavelength than butane.
(d) Ethanol and diethyl ether
Ethanol and diethyl ether are both polar organic compounds. However, diethyl ether is more nonpolar than ethanol. This is because the ethyl group (-CH2CH3) is a nonpolar functional group. The more nonpolar a molecule is, the higher its energy levels are. Therefore, ethanol is expected to absorb at a longer wavelength than diethyl ether.
Ethylene and ethene are both alkenes and have the same functional group. However, ethene has a longer chain length than ethene, which means that it has more electrons. The more electrons a molecule has, the lower its energy levels are. Therefore, ethene is expected to absorb at a longer wavelength than ethylene. This is because the energy of a photon is inversely proportional to its wavelength, meaning that a longer wavelength photon has lower energy.
(b) Propene and propene-1-ol
Propene and propene-1-ol are both three-carbon molecules containing a double bond. However, propene-1-ol also has a hydroxyl group (-OH) attached to one of its carbon atoms. The hydroxyl group is a polar functional group, which means that it can interact with polar solvents and other molecules. This interaction can lower the energy of the molecule, meaning that propene-1-ol is expected to absorb at a longer wavelength than propene.
(c) Butane and 2-butene
Butane and 2-butene are both four-carbon molecules. However, butane is a saturated hydrocarbon, while 2-butene is an unsaturated hydrocarbon. Saturated hydrocarbons have only single bonds between their carbon atoms, while unsaturated hydrocarbons have at least one double bond between their carbon atoms. Double bonds are more polar than single bonds, which means that they can interact with polar solvents and other molecules more strongly. This interaction can lower the energy of the molecule, meaning that 2-butene is expected to absorb at a longer wavelength than butane.
(d) Ethanol and diethyl ether
Ethanol and diethyl ether are both polar organic compounds. However, diethyl ether is more nonpolar than ethanol. This is because the ethyl group (-CH2CH3) is a nonpolar functional group. The more nonpolar a molecule is, the higher its energy levels are. Therefore, ethanol is expected to absorb at a longer wavelength than diethyl ether.
0