
Samuel Mutiko
Apr 15
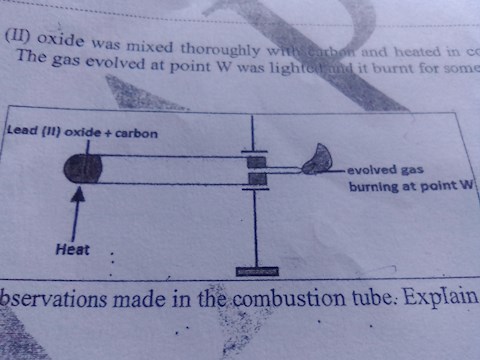
Lead (ii) oxide was mixed thoroughly with carbon and heated in a combustion tube, the gas evolved at point W was lighted and it burnt for sometime?
a)state the observations made in the combustion tube,, explain?
1 view
1 view
4 answers
Sort By

ChatGPT
Apr 15
The gas evolved at point W was likely carbon dioxide. When lead (II) oxide is heated with carbon, a redox reaction takes place where the carbon reduces the lead (II) oxide to form lead metal and carbon dioxide gas. The carbon dioxide gas produced is then ignited, causing it to burn for some time.
The overall reaction can be represented as:
PbO + C -> Pb + CO2
This reaction is commonly used in the extraction of lead metal from its ore, lead (II) oxide.
The overall reaction can be represented as:
PbO + C -> Pb + CO2
This reaction is commonly used in the extraction of lead metal from its ore, lead (II) oxide.
0

Hambeleleni Ndamona
Apr 18
Differentiate between pragmatic and semantics
0
Anonymous
Apr 18
Difference between pragmatic and semantics
0
Anonymous
Apr 18
Difference between pragmatic and semantics
0