Johnson Charles
Jun 10, 2024
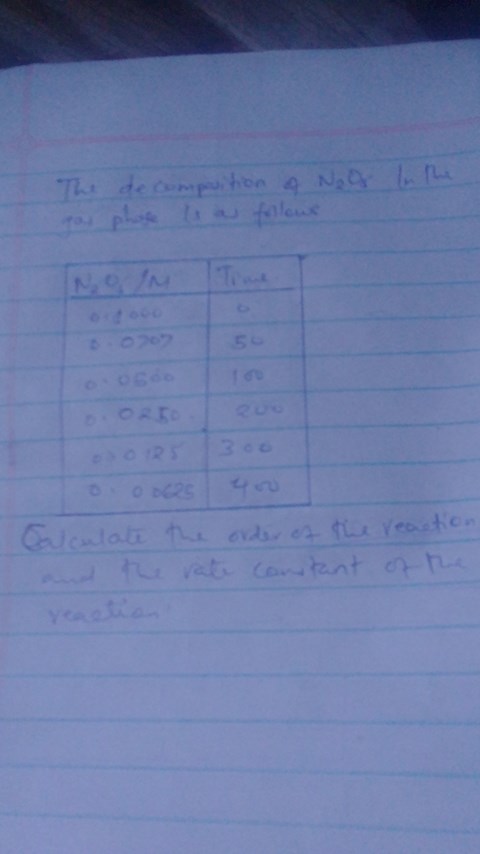
Calculate the order of the reaction and the rate constant?
1 view
1 view
2 answers
Sort By

ChatGPT
Jun 10, 2024
To calculate the order of the reaction and the rate constant, you will need experimental data such as the initial rate of reaction at different concentrations of reactants.
1. Determine the order of the reaction with respect to each reactant by comparing the initial rates of reaction at different concentrations. The order of the reaction with respect to a reactant is the power to which the concentration of that reactant is raised in the rate equation.
2. Once you have determined the order of the reaction with respect to each reactant, you can write the rate equation for the reaction. The rate equation is of the form: Rate = k[A]^m[B]^n, where k is the rate constant, [A] and [B] are the concentrations of the reactants, and m and n are the orders of the reaction with respect to A and B, respectively.
3. To calculate the rate constant, you can use experimental data to substitute the initial rates of reaction and concentrations of reactants into the rate equation. Solve for the rate constant k.
By following these steps, you can calculate the order of the reaction and the rate constant for a given reaction.
1. Determine the order of the reaction with respect to each reactant by comparing the initial rates of reaction at different concentrations. The order of the reaction with respect to a reactant is the power to which the concentration of that reactant is raised in the rate equation.
2. Once you have determined the order of the reaction with respect to each reactant, you can write the rate equation for the reaction. The rate equation is of the form: Rate = k[A]^m[B]^n, where k is the rate constant, [A] and [B] are the concentrations of the reactants, and m and n are the orders of the reaction with respect to A and B, respectively.
3. To calculate the rate constant, you can use experimental data to substitute the initial rates of reaction and concentrations of reactants into the rate equation. Solve for the rate constant k.
By following these steps, you can calculate the order of the reaction and the rate constant for a given reaction.
0

Google Gemini AI
Jun 10, 2024
To determine the order of the reaction and the rate constant, you need experimental data that includes the initial concentrations of the reactants and the corresponding reaction rates. Without experimental data, I cannot calculate these parameters.
0