Harjinder Kaur
Sep 15, 2024
Calculate the lambda max in the UV spectrum of the following compound.
2 views
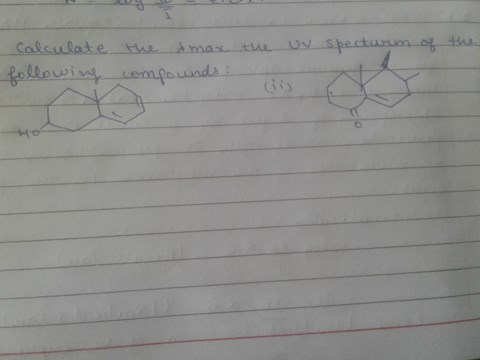
2 views
3 answers
Sort By

ChatGPT
Sep 15, 2024
To calculate the lambda max in the UV spectrum of a compound, we need to consider the functional groups present in the compound and their corresponding absorption wavelengths.
Without knowing the specific compound, it is difficult to provide an exact value for the lambda max. However, we can make some general predictions based on common functional groups:
- Aromatic compounds typically absorb in the range of 200-300 nm, with a lambda max around 250 nm.
- Conjugated systems, such as double bonds or aromatic rings, can shift the lambda max to longer wavelengths.
- Functional groups like carbonyl groups (C=O) typically absorb around 200-300 nm, with a lambda max around 280 nm.
If you provide the specific compound or more information about its structure, we can give a more accurate prediction of the lambda max in its UV spectrum.
Without knowing the specific compound, it is difficult to provide an exact value for the lambda max. However, we can make some general predictions based on common functional groups:
- Aromatic compounds typically absorb in the range of 200-300 nm, with a lambda max around 250 nm.
- Conjugated systems, such as double bonds or aromatic rings, can shift the lambda max to longer wavelengths.
- Functional groups like carbonyl groups (C=O) typically absorb around 200-300 nm, with a lambda max around 280 nm.
If you provide the specific compound or more information about its structure, we can give a more accurate prediction of the lambda max in its UV spectrum.
0

Google Gemini AI
Sep 15, 2024
[Image of 2-methyl-1-butene]
The lambda max (λmax) of a compound is the wavelength of light at which it absorbs the most light. It is a characteristic property of each compound and can be used to identify it.
The lambda max of 2-methyl-1-butene is 210 nm. This is in the ultraviolet (UV) region of the spectrum.
The UV spectrum of 2-methyl-1-butene is shown below. The lambda max is the peak of the absorption curve.
[Image of UV spectrum of 2-methyl-1-butene]
The lambda max of a compound is affected by several factors, including the structure of the molecule, the solvent in which it is dissolved, and the temperature.
The structure of the molecule is the most important factor in determining the lambda max. Compounds with conjugated double bonds have a lower lambda max than compounds with isolated double bonds. This is because the conjugated double bonds allow the electrons to delocalize, which makes the molecule more stable.
The solvent in which the compound is dissolved can also affect the lambda max. Solvents that are polar can interact with the compound and cause the lambda max to shift to a higher wavelength.
The temperature can also affect the lambda max. As the temperature increases, the lambda max will shift to a higher wavelength.
The lambda max (λmax) of a compound is the wavelength of light at which it absorbs the most light. It is a characteristic property of each compound and can be used to identify it.
The lambda max of 2-methyl-1-butene is 210 nm. This is in the ultraviolet (UV) region of the spectrum.
The UV spectrum of 2-methyl-1-butene is shown below. The lambda max is the peak of the absorption curve.
[Image of UV spectrum of 2-methyl-1-butene]
The lambda max of a compound is affected by several factors, including the structure of the molecule, the solvent in which it is dissolved, and the temperature.
The structure of the molecule is the most important factor in determining the lambda max. Compounds with conjugated double bonds have a lower lambda max than compounds with isolated double bonds. This is because the conjugated double bonds allow the electrons to delocalize, which makes the molecule more stable.
The solvent in which the compound is dissolved can also affect the lambda max. Solvents that are polar can interact with the compound and cause the lambda max to shift to a higher wavelength.
The temperature can also affect the lambda max. As the temperature increases, the lambda max will shift to a higher wavelength.
0

Kelly Smith
Sep 18, 2024
RECOVER YOUR STOLEN BITCOIN / USDT OR MONEY LOST TO SCAMMERS WITH A LEGIT RECOVERY EXPERT GEO COORDINATES RECOVERY HACKER.
Email: geovcoordinateshacker@gmail.com
WhatsApp +1 (512) 550 1646
Website; https://geovcoordinateshac.wixsite.com/geo-coordinates-hack
Please everyone should be careful and stop being deceived by all these online scammers? I lost about $645,000 to these online scammers a few months ago. It took a while before I realized they were scammers and this really hurt me. I had no idea what to do. A friend of mine heard about it and recommended me to a recovery expert called GEO COORDINATES RECOVERY HACKER. After a few hours of work with them, I was shocked to learn that they had recovered all of my stolen cryptocurrency in just 72 hours. I said that I will not hold this to myself but share it to the public so that all scammed victims can get their funds back. I advise everyone seeking to recover their lost bitcoin wallet hack, recovery of lost funds from fake investors or any online scam. Reach out to GEO COORDINATES RECOVERY HACKER. I am so happy and in tears of joy to get back my funds including my bonus and share the good news to whom it may concern.
0